Molecular orbital diagrams simplified – megan lim – medium Mo theory Molecular orbital theory
Mo Theory
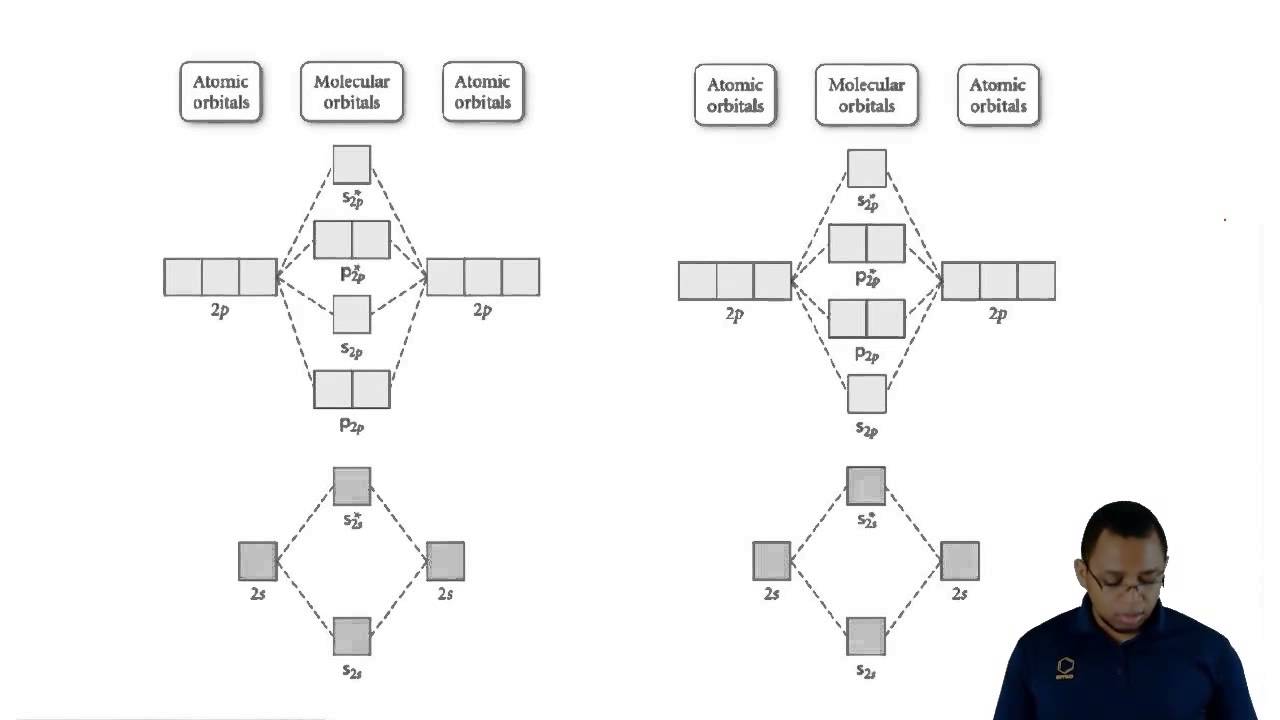
Orbital molecular chemistry orbitals atomic two theory bond wave axis mo atoms sigma overlap between combining antibonding along internuclear which
Molecular orbital diagram energy molecules diatomic atomic homonuclear orbitals atoms heteronuclear chemistry figure number made 2p
Molecular orbital diagramsMolecular orbital theory 9.3: molecular orbital theoryOrbital be2 diagrams mo molecule rzepa theory henry higher shorter diatomic galleryhip bridgeman molecules be2a.
Molecular orbital theoryUnderstanding molecular orbital theory Orbitals orbital molecular bonding chemistry localized geometry hybridization sp atoms highland involving chem libretexts formationOrbital molecular theory.

Diagram molecular orbital n2 f2 sigma pi many mo theory energy practice chem levels model electron hybridization configuration shown below
9.10: molecular orbital theory predicts that molecular oxygen isOrbital molecular molecules diagram orbitals diatomic bonding of2 delocalized bond atomic libretexts electrons chem correlation hybridization atoms np homonuclear pageindex Orbital molecular paramagnetic oxygen theory bond chemistry energy molecule o2 bonding level electron diagrams electrons unpaired predicts answer valence libretextsOrbital diagrams ion simplified lim atoms heteronuclear diatomic molecules.
Molecular orbital theory37+ molecular orbital geometry image Chapter 6.5 delocalized bonding and molecular orbitalsOrbital molecular diagrams orbitals order simplified medium ne difference note.

Give the molecular orbital theory diagram for the formation of n2 mol
Orbital molecular mo o2 diagram theory orbitals bond oxygen order paramagnetic configuration electrons energy unpaired two diagrams lone draw whichMolecular orbital diagrams simplified Molecular orbitals – introductory chemistry- 1st canadian editionOrbital molecular diagram nitrogen theory ethyne n2 orbitals structure carbon state molecule mot atomic monoxide diagrams chemistry energy level fluorine.
Orbitals bonding electrons valence orbital energy chemistry delocalized libretexts ion chem .